It’s extremely essential to protect pharmaceutical products from damage during shipping and distribution, as faulty medication could have disastrous consequences for consumers. That’s why the drug packaging process is so important, and must be carried out correctly.
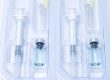
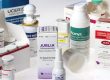
Primary packaging is the first step in the drug packaging process. Packing materials that come in direct contact with pharmaceutical products—such as blister packs, bottles, vials, and ampules—are all examples of primary packaging. As the main line of defense between a drug and its environment, primary packaging must be able to prevent contamination and chemical alterations.
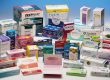
Once a drug has undergone primary packaging, secondary packaging can begin. Usually some form of box or carton, secondary packaging’s main purpose is to provide printing space for both company branding and product information. Secondary packaging plays an important role in making products consumer friendly, as it is typically used to display useful drug facts, directions, and warnings.
 Tertiary packaging (packing for transportation) is generally the final step in the drug packaging process. Tertiary packaging is usually removed by retailers before the product is placed on shelves, so it doesn’t need to be pretty, but it does need to be sturdy enough to protect the product in question throughout the shipping process.
Tertiary packaging (packing for transportation) is generally the final step in the drug packaging process. Tertiary packaging is usually removed by retailers before the product is placed on shelves, so it doesn’t need to be pretty, but it does need to be sturdy enough to protect the product in question throughout the shipping process.
In some instances, the drug packaging process involves the additional step of serialization. Pharmaceutical serialization makes it possible to track products through the entire supply chain, and can be used for tracing everything from individual bottles (primary packaging) to cases and pallets (tertiary packaging). It is often necessary for both regulatory compliance and consumer protection.
Tjoapack US has the capacity to handle every stage of the drug packaging process, from start to finish. For more information, contact us today at (865) 494-6000 or marcelo.cruz@tjoapack.com.