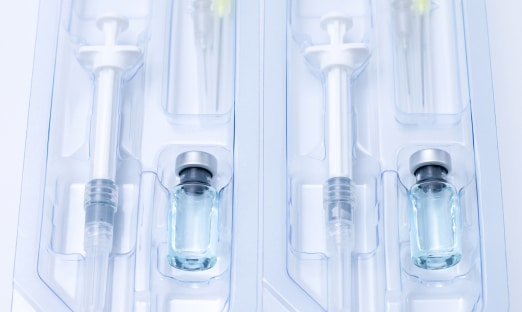
Medical device packaging plays a vital role in the public health sector, ensuring that medical devices make it to consumers without being damaged or compromised. With such an important job to do, medical device packaging must meet several strict requirements.
Material
All medical device packaging should be manufactured using materials that are both known and traceable. Additionally, the materials used should be non-toxic, odorless, and non-leaching to prevent any possible contamination of the device the packaging is designed to contain. When choosing a packaging company, make sure they offer the appropriate materials for your application before making any final decisions.
Durability
One of the primary functions of medical device packaging is protecting the product in question from sustaining physical damage as it moves through the supply chain. It needs to be durable enough to withstand every stage of the shipping process, and every climate to which it may be exposed. If a piece of material has holes, tears, cracks, or creases, it is not fit for use in any kind of medical device packaging.
Integrity
Some medical devices may remain in storage or on shelves for a prolonged period of time before being purchased and used. In light of this, medical device packaging must be able to maintain its integrity in the long term. When working with a medical device packaging company, make sure to discuss packaging service life to ensure they can meet your specific needs.
At Tjoapack US, we provide a variety of medical device packaging services, including vial and ampule labeling, pre-filled syringe packaging, and pharmaceutical kit assembly. We are also cGMP, FDA, and DEA compliant. If you would like to learn more about our medical device packaging solutions, contact us today. Give us a call at (865) 494-6000 or send an email to solutions@tjoapack.com. We look forward to assisting with all of your packaging needs.